Medical Device Cybersecurity
Humanizing and Simplifying Product Security for Medical Device Manufacturers and Engineers
End-to-End Support
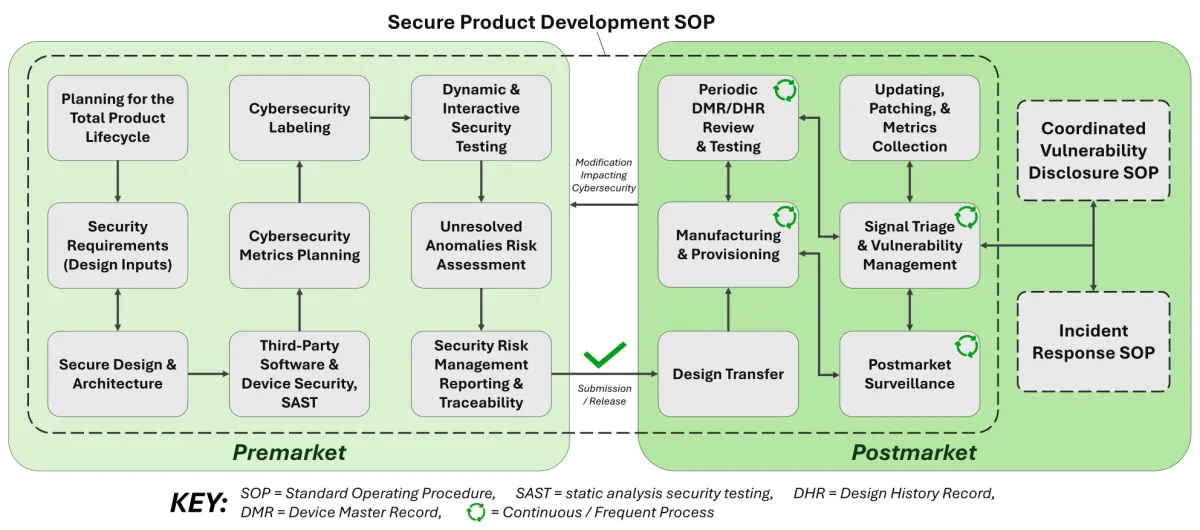
We can perform every activity and provide every document as required by global cybersecurity guidance for device submissions and long-term sustaining efforts, including governance, threat modeling, SBOMs, and full testing services.
Governance
Policies and procedures incorporated into your QMS compliant with global standards and regulations.
Security Planning
Security risk management planning for the total product life cycle, from requirements through end of life.
Security Requirements
Support with system and product / specification level security requirements that are traceable and testable.
Secure Design & Architecture
Threat modeling of assets and processes with crafted controls, risk assessments, and security architecture views.
Third-Party & Supply Chain
Software Bill of Materials (SBOM) with vulnerability analyses, support information, and vendor assessments.
Cybersecurity Metrics
Metrics to be gathered during update and patch events, provided as a required eSTAR artifact.
Cybersecurity Labeling
Content to be added to the IFU and user manual for transparency to end users.
Security Testing
System-wide vulnerability testing and penetration testing by qualified experts with independence.
Unresolved Anomalies
Security assessments of software bugs following Verification and Validation activities.
Reporting & Traceability
Submission-ready artifacts to be included in the DHF according to our templates and compliant with global expectations.
Postmarket Compliance
Sustaining DMR/DHR review, periodic testing, SBOM & other monitoring, COTS device administration, and incident response & disclosure support.
Bolster in-house capabilities with 3 levels of training for executives, quality & regulatory, and developers & engineers.
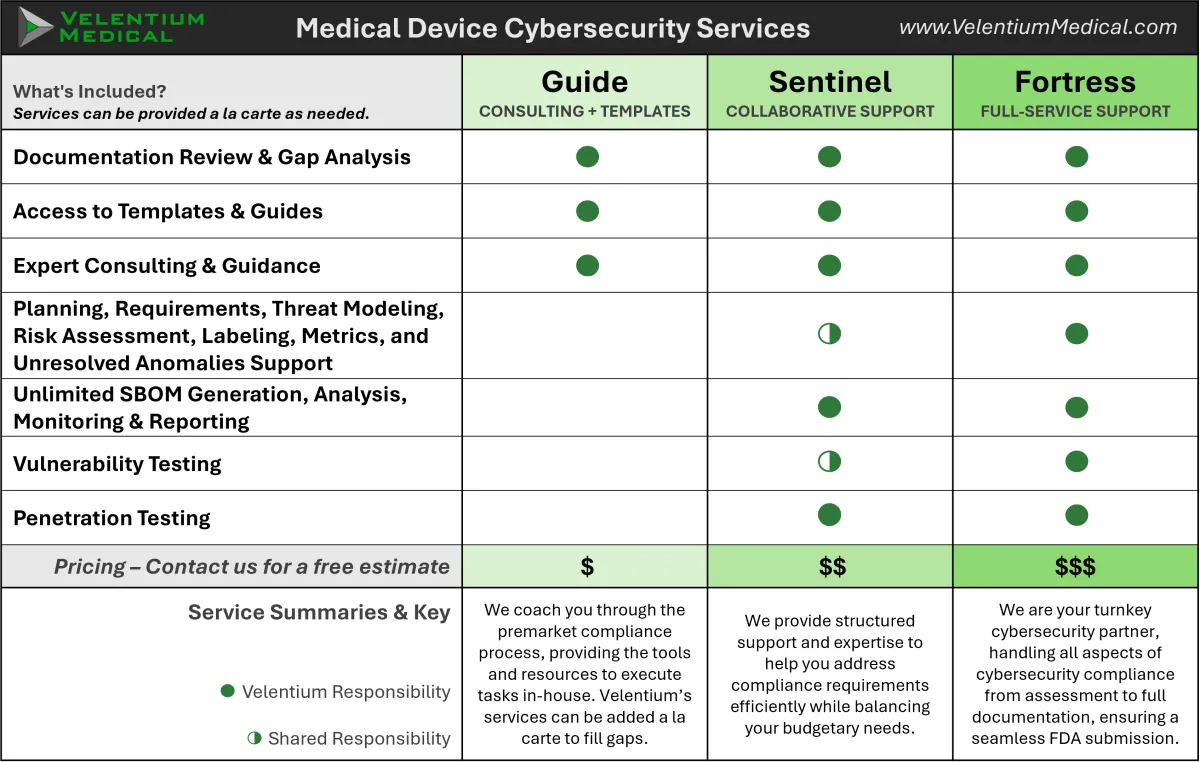
Flexible Cybersecurity Services for Every Need
Whether you need targeted guidance, collaborative support, or a fully outsourced security division, we adapt our services to your project’s scope, team size, and budget.
Wherever you are in your product life cycle, we can help.
Meet Our Experts
With decades of safety-critical cybersecurity experience, Velentium Medical offers best-in-class product security services. Learn from our experts by visiting our Resource Center.
Safe. Secure. Effective.
One stop for secure Medical Device R&D, product development, contract
manufacturing, and postmarket services
Who We Are
© 2026 Velentium, LLC. All Rights Reserved.